|
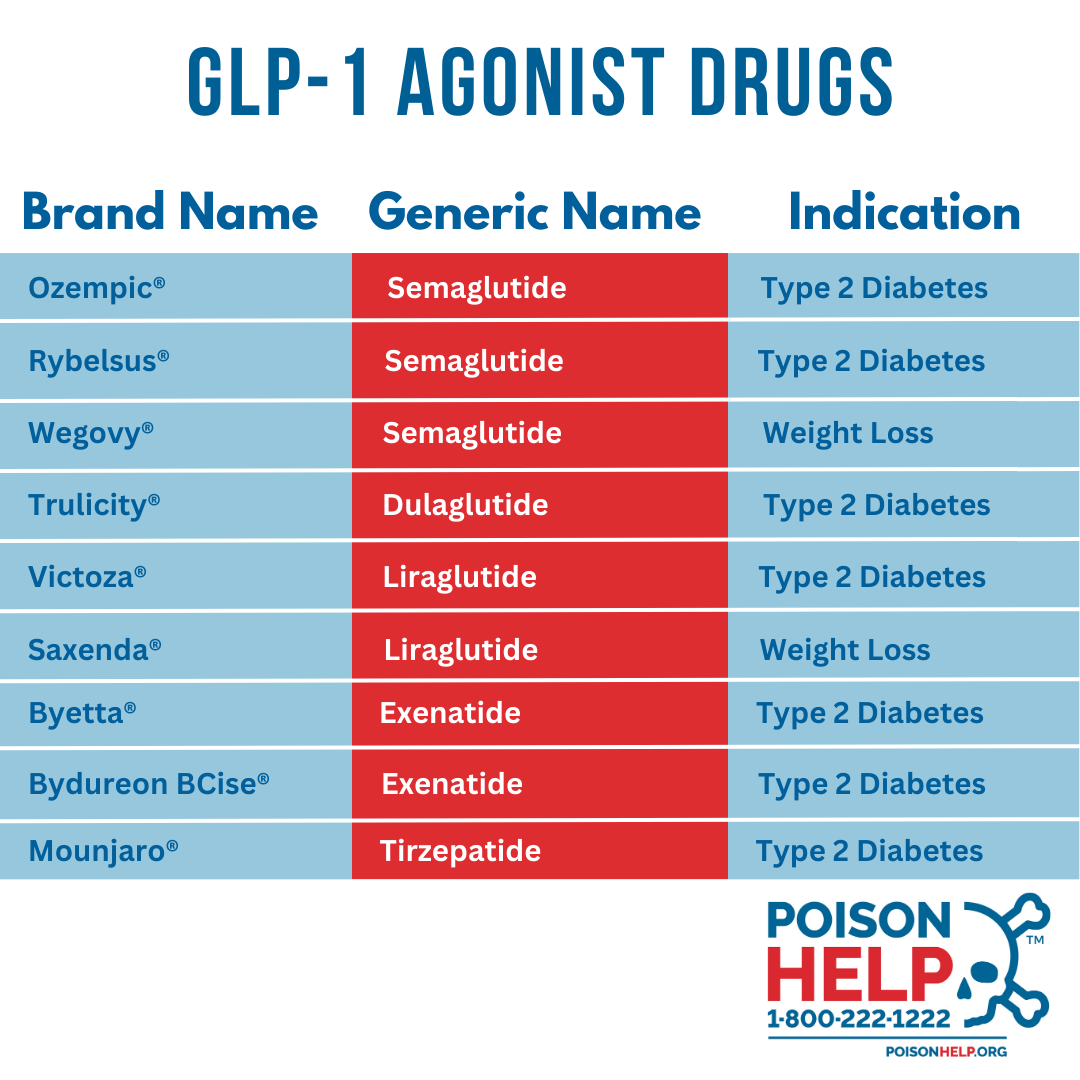
What Are GLP-1 Drugs? GLP-1, or glucagon-like peptide-1, is a naturally occurring hormone in the body that plays a crucial role in regulating blood sugar, appetite, and digestion. In recent years, synthetic versions of this hormone have emerged. These drugs that are FDA-approved for the treatment of diabetes have emerged as a promising new tool in the fight against obesity. Examples include Trulicity®, Ozempic®, Rybelsus®, Wegovy®, and many others. GLP-1 agonists mimic the effects of the body’s natural hormone by reducing appetite, slowing down the rate at which food leaves the stomach, and offering a slight boost in metabolism (which helps burn more calories at rest.)
Why are GLP-1 Agonist Overdose Trending? Poison Centers have seen a nearly 1,500% increase in calls since 2019 related to overdose or side effects of injectable weight-loss drugs. Poison center calls related to GLP-1 drugs are increasing due to their popularity and increased use for weight loss. The high demand of GLP-1 drugs has resulted in shortages making it challenging for patients to access the medication through their regular pharmacy. This has led to patients seeking access to these medications through online companies or compounding pharmacies. While it is legal for compounding pharmacies to produce GLP-1 drugs on shortage, the products are not reviewed for safety or efficacy by FDA. FDA reported that some compounded pharmacies used different salt forms of semaglutide, which are different from the active ingredient found in FDA approved semaglutide products. Finally, there have been reports of counterfeit Ozempic entering the U.S. marketplace. Counterfeit products are different from compounded drugs as they are illegally manufactured, distributed, and are often contaminated with undeclared active ingredients. These products may be sold illegally online. Poison Centers are most often contacted about accidental therapeutic errors to FDA- approved GLP-1 drugs. Common mistakes include taking doses too closely together or taking a higher than recommended dose at once. With compounded products, patients report accidentally taking 10-times the recommended dose due to confusing measurement units while using a syringe. As of April 30, 2025, Poison Centers have managed 3,633 GLP-1 agonist related exposure cases. From 2019 to 2025, Poison Centers have managed 22,966 GLP-1 agonist related exposure cases. What are the Signs and Symptoms of a GLP-1 agonist poisoning? It is important to note that GLP-1 drugs are prescription medications and should only be taken under the supervision of a healthcare professional. Some of the more common side effects include:
Overdose signs and symptoms are similar to side effects, however, the duration of symptoms may last longer. In severe cases, dehydration or hypoglycemia (especially if used with certain drugs like insulin or sulfonylureas) can occur. Take Action For questions about GLP-1 medications and overdoses, call Poison Help at 1-800-222-1222. From prescription-related questions to concerns about the signs and symptoms of a potential overdose, our poison control experts are ready and available, 24/7/365, free of cost, for emergencies and non-emergencies. You can also get immediate online support and recommendations through our Get Help tool. For the Media Please cite this data as “National Poison Data System, America's Poison Centers.” Any and all print, digital, social, or visual media using this data must include the following: “You can reach your local Poison Center by calling the Poison Help line: 1-800-222-1222. To save the number in your mobile phone, text POISON to 301-597-7137.” Email media@PoisonCenters.org or call 703-894-1863 for more information, questions, or to submit a data request. | PREVENTIONIt’s important to take all medication exactly as prescribed. For GLP-1 drugs, this is particularly important. Here are some tips to help avoid overdoses:
|
Important notes about Poison Center dataAmerica's Poison Centers maintains the National Poison Data System (NPDS), the national database of information logged by the country’s Regional Poison Centers serving all 50 United States, Puerto Rico, the District of Columbia, and territories. Case records in this database are from self-reported calls: they reflect only information provided when the public or healthcare professionals report an actual or potential exposure to a substance, request information, or request educational materials. As such:The term “exposure” means someone has had contact with the substance in some way; for example, ingested, inhaled, or absorbed a substance by the skin or eyes, etc. Exposures do not necessarily represent poisonings or overdoses. |
/APC%20logo_2C%20with%20tagline.jpg)
 Glucagon-like Peptide-1 (GLP-1) Agonists
Glucagon-like Peptide-1 (GLP-1) Agonists